Research
Outline of our laboratory’s research
Our bodies and most of the things around us are all made of molecules, ions, and their composite materials. However, they are so small that they are invisible to the eye and difficult to observe directly. In our laboratory, we are studying methods to convert these behaviors into measurable signals such as light and electricity by taking advantage of the properties of molecules and ions. Specifically, after understanding the size, shape, polarity, and other properties of the target molecule, we create a “trick” to recognize the molecule, for example, to light up only when the molecule is adsorbed. If the device succeeds in working, the movement of the molecule to be measured will be visualized as light or electricity, enabling “chemical analysis” or “sensing” to confirm the presence of a specific substance or to track reactions. Since this research can target a wide variety of substances, it is expected to be applied to a very wide range of fields, including disease testing, environmental monitoring of air and water, detection of pollutants and toxic substances, and quality control of food and medicine.
Electrochemical Analysis: Taking Advantage of the Unique Electrochemical Properties of Monolayer Graphene
【一山ら「支持膜担持ポリエチレンイミン修飾単層グラフェン電極を用いた電気化学測定」 日本分析化学会第73年会(2024).】
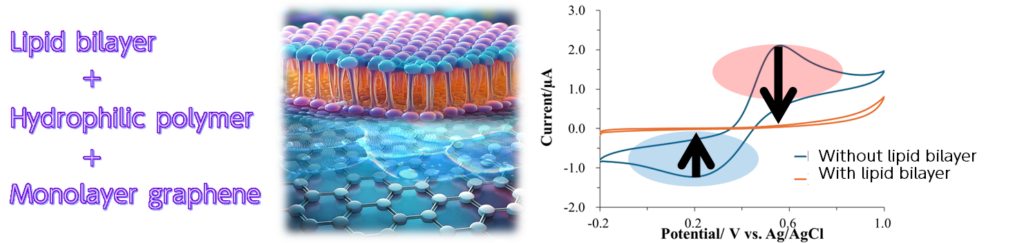
We have successfully fabricated electrodes with lipid bilayers on the surface of hydrophilic polymer-modified single-layer graphene. The lipid bilayer is an insulating membrane that does not react with electrochemical species present in the system. When pore-forming toxins such as antimicrobial agents are present, the electrochemical species permeate the membrane and react with them, leading to detection.
【Ryohei Suzuki et al., “Enhancement of Electrochemical Charge Transfer in the Reduction of Hexacyanoferrate(III)/(IV) by DPPZ Adsorbed on Monolayer Graphene Surface” MRM2023/IUMRS-ICA2023.】
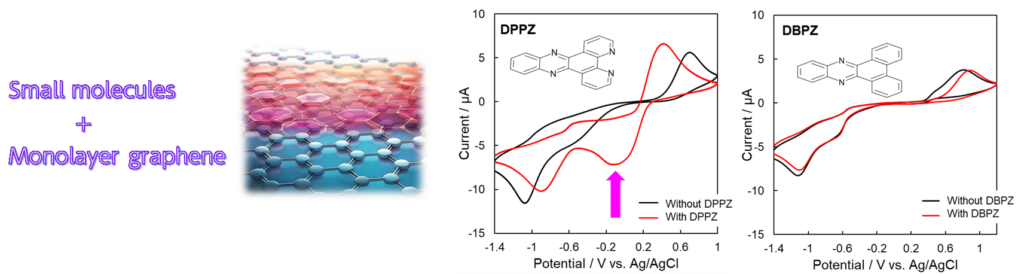
As a property unique to monolayer graphene electrodes, when measuring the redox reaction of [Fe(CN)6]3-/4-, we found that the addition of small molecule DPPZ containing pyridine-type nitrogen structure in solution activates the reaction, particularly increasing the reduction peak. The addition of small molecule DBPZ of approximately the same size, which does not contain pyridine-type nitrogen structure, does not change the reaction efficiency.
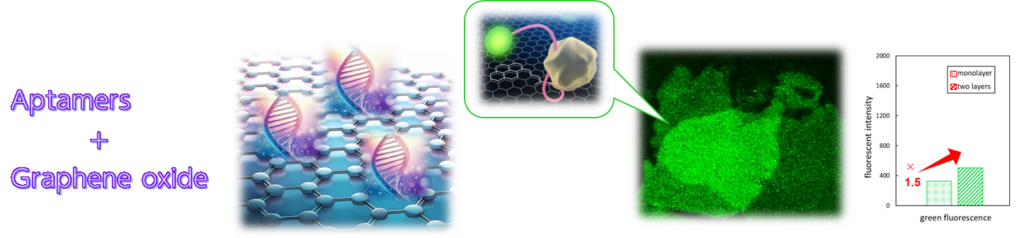
Fluorescence Analysis: Taking Advantage of Interactions between Graphene Oxide and Biomolecules
【木島ら「蛍光型酸化グラフェンバイオセンサの積層数と還元状態に依存する応答特性の変化」 日本分析化学会第72年会(2023).】
By modifying graphene oxide surfaces with molecular recognition elements consisting of single-stranded DNA aptamers labeled with fluorescent molecules, a biosensor can be constructed in which fluorescence is observed only when a target molecule that specifically binds to the aptamers is present due to graphene oxide’s special optical properties. We have found that as the number of graphene oxide layers increases, the observed fluorescence intensity increases and the sensitivity becomes higher.
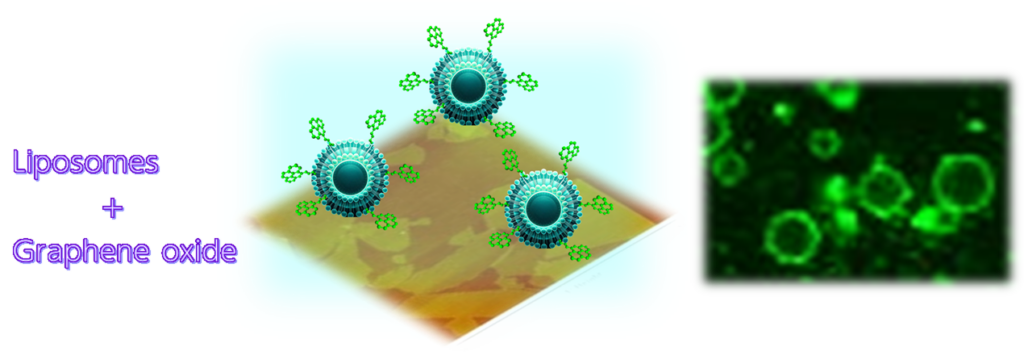
【兼子ら「ピレン骨格で表面機能化された巨大ベシクルとグラフェン表面との相互作用」 日本分析化学会第72年会(2023).】
When lipid molecules containing pyrene, a small aromatic molecule, are mixed with the lipid bilayer that constitutes the wall of a spherical vesicle liposome and interact with a graphene oxide-anchored substrate, the liposome is adsorbed by the π-π interaction between the pyrene and graphene oxide and is stabilized to the extent that it does not fall even when the substrate is inverted. The liposome can be stably fixed to the substrate even when the substrate is inverted. In the future, we aim to apply this technology to the transport of chemical substances encapsulated in liposomes.